14 May Weekly Update: CDC Health Advisory States Flu Season At Epidemic Levels
NEXTGEN LABORATORIES WEEKLY INFLUENZA UPDATE
A Special Health Advisory from the CDC regarding this year’s dominant strain (H3N2) and guidance to clinicians on the selection of antiviral medications.
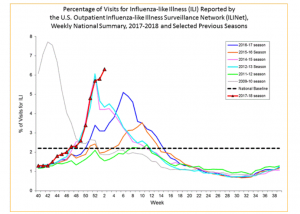
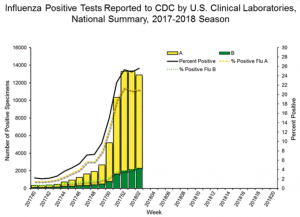
Jan 23, 2017 -As was the case last week in NextGen’s flu report and, the latest data in from the CDC shows that influenza activity has continued to increase, with every state now experiencing widespread outbreaks and predictions that the severe influenza season will continue for another 12 weeks. Of concern to all hospitals, physician offices and urgent care centers is that mortality rates from secondary infections, primarily pneumonia in the elderly, are now at epidemic levels (chart below).
Special Health Advisory released by the CDC again this week includes:
- a notice about increased influenza A(H3N2) activity and its clinical implications;
- a summary of influenza antiviral drug treatment recommendations;
- an update about approved treatment drugs and supply this season; and
- background information for patients about influenza treatment.
More information is available at https://emergency.cdc.gov/han/han00409.asp.
More on Influenza Strains and Antiviral Recommendations for Clinicians:
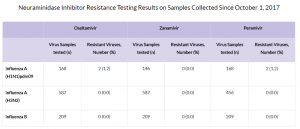
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications.
Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.

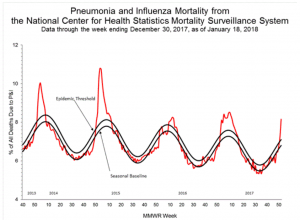
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on January 18, 2018, 8.2% of the deaths occurring during the week ending December 30, 2017 (week 52) were due to P&I. This percentage is above the epidemic threshold of 7.1% for week 52.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. Due to the additional time needed for manual coding, the initially reported P&I percentages may be lower than percentages calculated from final data. Previous longer backlogs in manual coding have been resolved and death records are now coded within 10 days from receipt of a death record by NCHS.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
It is more important than ever to identify the causative pathogens of infection – not just for the flu, but to know what other respiratory illnesses you might be dealing with since many patients walk out of clinician offices and Urgent Care Settings with negative rapid immunoassay flu tests yet might have other serious viral or bacterial respiratory illnesses as has been the case in a number of cases that made national news where young people died from pneumonia or complications from co-infections. NGL’s rapid diagnostic testing will help you make good, informed decisions related to the use of antivirals in the critical 48 hour of efficacy, whether to use antibiotics or not and if patients in community settings need to be isolated.
Benefits of the NextGen rapid infection diagnostic tests include:
– Identify causative pathogens of the infection with over 95% sensitivity and specificity compared to 50% or less for Rapid Flu Tests (RIDT’s)
– Determine appropriate antibiotic use or if antibiotic use is necessary
– Identity within hours instead of days causative agents of infection
– Support you in following the latest mandates by the CDC and CMS for infection prevention and control and antibiotic stewardship
– For more information or questions regarding these powerful tools to support your fight against flu season please reach out to your NGL sales representative
Graphs and data courtesy of CDC Flu View: https://www.cdc.gov/flu/weekly/index.htm#S1


